Alzheimer’s disease research is spearheading innovative breakthroughs in our understanding of neurodegenerative diseases, fundamentally altering how we view the brain’s immune system. Pioneered by leading scientists like Beth Stevens, this field focuses on the crucial role of microglial cells, which are responsible for maintaining brain health by pruning synapses and clearing out cellular debris. Stevens’ groundbreaking studies at Boston Children’s Hospital have revealed that abnormal microglial activity can contribute to cognitive decline, affecting millions of Americans diagnosed with Alzheimer’s. Furthermore, her work has enhanced the search for biomarkers for Alzheimer’s, opening doors to potential treatments that could reshape the landscape of neurological care. As we delve deeper into the mechanisms of brain health, the insights gained from Stevens’ research may lead to revolutionary strategies to combat these debilitating diseases.
Exploring the frontiers of cognitive health, Alzheimer’s disease research encompasses a vital inquiry into the mechanisms underlying neurodegenerative disorders. This area of study, led by experts like Beth Stevens, investigates how the brain’s immune defenders—microglial cells—play a fundamental role in the delicate balance of neuronal communication and damage repair. Recognizing the significance of abnormal synaptic pruning in these conditions not only shifts our comprehension of Alzheimer’s but also emphasizes the need for identifying effective biomarkers for early detection. As research in this sphere advances, the potential for developing targeted therapies grows, promising a brighter future for those affected by Alzheimer’s and other memory-related ailments. Ultimately, this scientific pursuit is pivotal for enhancing healthcare solutions and improving the lives of millions.
Understanding Microglial Cells and Alzheimer’s Disease
Microglial cells are a crucial component of the central nervous system, functioning as the brain’s innate immune cells. They play a significant role in maintaining homeostasis, clearing cellular debris, and modulating synaptic connections. In recent research conducted by Beth Stevens and her team, these cells have been identified as potential culprits in the pathogenesis of Alzheimer’s disease. Abnormalities in their pruning of synapses, which are critical for neural communication, may lead to neuron degeneration and cognitive decline associated with Alzheimer’s.
Research into microglial cells has expanded our understanding of how they can contribute to neurodegenerative diseases such as Alzheimer’s and Huntington’s. The balance in their role—ensuring proper synaptic pruning without overreacting—is crucial. When microglial cells malfunction, as seen in the context of Alzheimer’s disease, the consequences can be dire. Identifying biomarkers for Alzheimer’s that reflect unhealthy microglial activity holds promise for early intervention and potentially therapeutic strategies.
The Role of Synaptic Pruning in Neurodegenerative Diseases
Synaptic pruning is a natural process that occurs in the brain during development and throughout life. It is essential for the elimination of unnecessary or weak synaptic connections, leading to refined neural circuits. However, when this process becomes dysregulated—as highlighted in studies by Beth Stevens—the consequences can be detrimental, especially in the context of neurodegenerative diseases like Alzheimer’s. This abnormal pruning can exacerbate the loss of neuronal connections, contributing to cognitive decline and memory loss.
Understanding the mechanisms behind synaptic pruning is vital for developing effective strategies to combat Alzheimer’s disease. Researchers are actively exploring how the misbehavior of microglial cells leads to excessive synaptic pruning. By identifying potential biomarkers for Alzheimer’s, scientists hope to establish new therapeutic targets that can mitigate the harmful effects of this aggressive pruning process, offering hope for millions affected by neurodegenerative diseases.
Innovations in Biomarkers for Alzheimer’s Disease Detection
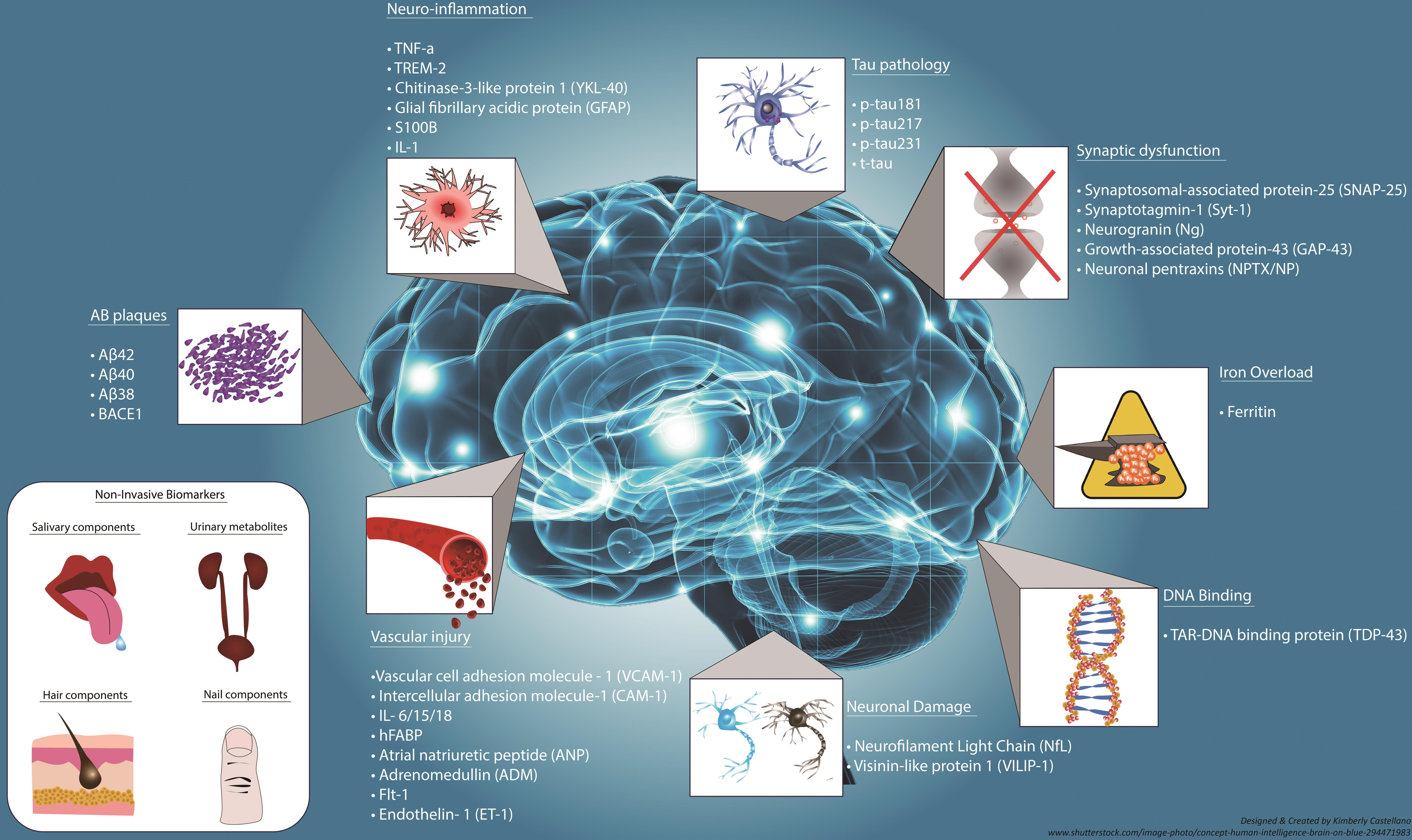
The quest for effective biomarkers for Alzheimer’s disease has been a focal point in recent research, particularly at the Stevens Lab. Biomarkers can provide invaluable insights into the progression of Alzheimer’s disease, allowing for earlier and more accurate diagnoses. With advancements in understanding microglial activity and synaptic pruning, researchers are uncovering potential indicators that reveal the presence of Alzheimer’s pathology before clinical symptoms even emerge.
The development of these biomarkers could revolutionize how practitioners monitor the onset of Alzheimer’s. Beth Stevens’ work emphasizes the connection between inflammatory responses in the brain and the pathological changes associated with neurodegenerative diseases. By refining these biomarkers, we can better track disease progression and response to treatment, ultimately improving patient outcomes and paving the way for personalized medicine in combating Alzheimer’s.
Beth Stevens: Pioneering Research in Neurodegenerative Disorders
Beth Stevens stands at the forefront of Alzheimer’s disease research, driven by her profound curiosity about the brain’s immune system and its interactions with neuronal networks. Her groundbreaking studies have illuminated the role of microglial cells in neurodegenerative disorders, reshaping how scientists understand and approach the pathology of diseases like Alzheimer’s. Her dedication exemplifies the impact of federal support, which has enabled her lab to explore complex interactions and uncover vital mechanisms underpinning these conditions.
Stevens’ work is not only about understanding the disease but also about inspires the next generation of researchers in neuroscience. Her vision aligns with the growing recognition of the importance of integrating basic science with clinical applications. As highlighted in her research, tracking the behavior of microglial cells could yield new insights into therapeutic strategies, making her work a cornerstone for future innovations in Alzheimer’s disease treatment.
The Impact of Curiosity-Driven Research on Neurology
Curiosity-driven research has been central to the advancements we see in neurological sciences today. It allows scientists like Beth Stevens to explore uncharted territories, connecting seemingly unrelated findings into a coherent understanding of complex disorders such as Alzheimer’s disease. By delving into the mechanisms of microglial cells and synaptic pruning, Stevens has paved the way for new frameworks of understanding and tackling neurodegenerative diseases.
The implications of her research extend beyond academia; they touch the lives of millions dealing with Alzheimer’s disease. The insights gained from such explorative research play a crucial role in shaping future therapies. Innovations stemming from curiosity-driven approaches can lead to breakthroughs that translate fundamental discoveries into practical solutions for patients, creating a ripple effect that enhances our collective fight against neurodegeneration.
Funding and Support in Alzheimer’s Research
The role of funding in Alzheimer’s research cannot be overstated. Beth Stevens often highlights the importance of support from organizations like the National Institutes of Health. Such funding not only fuels exploratory studies but also creates an environment where researchers can pursue innovative ideas without immediate commercial pressures. This support has been pivotal in developing new insights into microglial function and its implications for neurodegenerative diseases.
Moreover, sustained financial backing fosters collaborations across institutions such as the Broad Institute of MIT and Harvard, leading to multidisciplinary approaches that enhance research outcomes. As Stevens’ work illustrates, adequate funding is a lifeline for advancing our understanding of Alzheimer’s disease and refining strategies to combat its effects on millions of individuals. The journey from basic science to applied treatment hinges on this vital support.
Neuroscience and Neurodegenerative Disease Awareness
Increased awareness about neurodegenerative diseases such as Alzheimer’s is crucial for research and treatment. As scientists like Beth Stevens improve our understanding of microglial cells and their relation to synaptic pruning, the general public gains insight into the biological underpinnings of these complex conditions. By disseminating knowledge about neuroinflammation and its role in Alzheimer’s, awareness can lead to greater public support for funding and resources dedicated to research.
Additionally, raising awareness about the symptoms and progression of Alzheimer’s encourages early diagnosis, essential for beneficial interventions. Educational initiatives are vital to informing communities about the importance of recognizing warning signs and understanding the evolving nature of neurodegenerative diseases, fostering a proactive approach in combating these illnesses.
Future Innovations in Alzheimer’s Treatment Strategies
The future of Alzheimer’s treatment lies in the integration of cutting-edge research and innovative strategies. As researchers build on foundational studies regarding microglial cells, new therapies targeting neuroinflammatory pathways may emerge. This ongoing research effort aims not only to halt disease progression but also to restore cognitive functions compromised by conditions like Alzheimer’s.
With the potential for tailored therapeutic approaches based on specific biomarkers, treatments could be customized to fit individual patients, which may significantly improve their quality of life. As neuroscience continues to evolve through persistent research efforts, the dream of developing effective therapies for Alzheimer’s disease becomes increasingly attainable.
Collaboration Across Disciplines in Neuroscience
Collaboration across various scientific disciplines is at the heart of advancing Alzheimer’s research. When fields such as neurology, immunology, genetics, and psychiatry come together, it fosters a holistic understanding of diseases like Alzheimer’s. Researchers like Beth Stevens exemplify the benefits of multidisciplinary collaboration as they work closely with biologists and clinicians to translate laboratory findings into real-world applications.
This interconnectedness enables researchers to address complex questions surrounding neurodegenerative diseases effectively. Through sharing expertise and resources, scientists can harness collective knowledge to innovate treatment strategies and discover new therapies that target the multifaceted nature of Alzheimer’s disease, driving forward the battle against this debilitating condition.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease research?
Microglial cells are integral to Alzheimer’s disease research as they function as the brain’s immune system. They help clear out damaged cells and are involved in the process of pruning synapses, which is crucial for healthy brain function. Abnormal microglial activity can lead to neurodegenerative diseases, including Alzheimer’s, making them a key focus for developing biomarkers for Alzheimer’s and potential treatment options.
How does synaptic pruning relate to Alzheimer’s disease?
Synaptic pruning, the process by which unnecessary synapses are eliminated, is significant in Alzheimer’s disease research. In healthy brains, microglial cells manage this process, but in Alzheimer’s, abnormal pruning may contribute to the loss of synaptic connections. Understanding this mechanism is crucial for identifying biomarkers for Alzheimer’s and developing therapeutic interventions.
What discoveries has Beth Stevens made in Alzheimer’s disease research?
Beth Stevens, a prominent figure in Alzheimer’s disease research, has uncovered how microglial cells influence synaptic pruning and contribute to neurodegenerative diseases. Her work has identified potential biomarkers for Alzheimer’s, paving the way for new treatments aimed at improving care for patients affected by this disease.
How do researchers identify biomarkers for Alzheimer’s using microglial cells?
Researchers identify biomarkers for Alzheimer’s disease by studying microglial cells and their role in synaptic pruning. Abnormalities in the behavior of these immune cells may indicate the presence or progression of Alzheimer’s, allowing scientists to develop diagnostic tools and treatments targeting neurodegenerative diseases.
Why is basic science important for advancements in Alzheimer’s disease research?
Basic science is crucial for advancements in Alzheimer’s disease research because it lays the foundation for understanding complex mechanisms at play in neurodegenerative diseases. Investigations initiated through basic research, such as those by Beth Stevens on microglial cells, can lead to significant breakthroughs in developing biomarkers and therapies for Alzheimer’s.
What is the significance of abnormal synaptic pruning in neurodegenerative diseases?
Abnormal synaptic pruning is significant in neurodegenerative diseases like Alzheimer’s because it may lead to the loss of crucial neural connections. Research in this area, particularly focusing on microglial cells, helps scientists understand the pathological processes underlying Alzheimer’s, leading to potential interventions and biomarkers for early detection.
How do microglial cells affect neurodegenerative diseases like Alzheimer’s?
Microglial cells affect neurodegenerative diseases like Alzheimer’s by responding to cellular damage and maintaining synaptic health through pruning. When their function is disrupted, it can result in excessive pruning or inflammation, contributing to the progression of diseases such as Alzheimer’s. Therefore, studying these cells is vital for both understanding and treating neurodegenerative disorders.
| Key Points | Details |
|---|---|
| Beth Stevens’ Research | Focuses on microglial cells and their role in the brain’s immune system. |
| Microglial Functions | They patrol for illness, clear damaged cells, and prune synapses. |
| Implications for Alzheimer’s | Abnormal pruning by microglia can lead to Alzheimer’s and other disorders. |
| Impact on Treatment | Research aids in developing new biomarkers and medications for Alzheimer’s disease. |
| Funding Sources | Research supported primarily by NIH and federal funding. |
Summary
Alzheimer’s disease research is witnessing a significant evolution, particularly through the pioneering work of researchers like Beth Stevens. Her contributions to understanding the immune functions of microglial cells highlight the intricate link between the brain’s health and neurodegenerative diseases such as Alzheimer’s. By investigating how these cells operate, especially their influence on synaptic pruning, Stevens is uncovering vital pathways that could lead to innovative treatments. With an estimated 7 million Americans affected by Alzheimer’s, advancements in research like this are critical for developing effective interventions and improving patient care.